Download the Free eBook
All of the information contained on this page is available in a free, easy-to-use interactive PDF eBook. This guide will teach you best practices for testing pH of food and drink, as well as proper maintenance of pH meters and electrodes, including cleaning, calibration, and storage.
Introduction to Testing pH in Food
pH. What is it? And what’s its role in food? This guide is intended as primer for understanding the role of pH measurement in food and beverage. It will cover measuring of pH for purposes of ensuring food safety and achieving balance of flavor. It will outline the tools necessary for accurately testing pH, the different types of pH meters and electrodes, the importance of proper cleaning, calibration and storage, and proper methods for testing foods of varying composition.
My First pH Meter
My first experience with a pH meter was fist-through-the-wall aggravating. The meter was a Hanna HI98103 pH Tester . I was getting deeper into fermentation and needed something more reliable than test strips, but wasn’t quite ready to drop a few hundred dollars on a meter. Out of the box, the meter was great, but after a few uses, I was finding that it would drift. A sample of pickle brine would range from pH 4 to 5 and everything in between, never landing on a number. After a bit of research, I presumed that the issue was one of calibration, so I purchased calibration buffers. I calibrated to the first buffer, a 4.01, by twisting the little screw until it read “4.01”. The next buffer was a 7.01. I calibrated to it — and now the 4.01 is off again! Back to Google to figure out what hell I’m doing wrong. I finally determined that the electrode may be dirty. I did my best to clean it with water and dab it with a clean rag. The results were better but there always seems to be discrepancies and I never fully trusted the readings, so I eventually trashed the meter.
Years later, I realized the issue was one of user error. I know now that testing pH is more than buying a meter and sticking it in a pickle. pH electrodes are sensitive instruments and require careful cleaning, frequent calibration and proper storage. My issue then was one of cleaning and storage. My electrode was never properly cleaned; though it looked clean, there were still contaminants — like oil films from meat — that prevented accurate calibration. I also was not storing the electrode as I should. I was using hard tap water which was leaving mineral deposits on the electrode.
I often hear others relating anecdotes that echo my past frustration and bad experiences. There is a lot of information available on pH measurement, but much of it is contradictory and confusing; and little focuses specifically on its application in food. What follows is an attempt to provide insight and guidance on how to properly use and maintain a pH meter for purposes of testing food and drink.
Table of Contents
Jump to a particular topic of interest by using the table of contents below:
What is pH?
pH is the quantitative measurement of the hydrogen ion activity in a solution.
The hydrogen ion is the nucleus of a hydrogen atom, separated from its accompanying electron. The hydrogen nucleus is made up of a particle carrying a unit of positive electric charge, called a proton.1 The isolated hydrogen ion is represented by the symbol H+, which gives us the “H” in “pH”, where the “p” stands for “potential”.
Acid concentration vs. pH
It is a common misconception that pH measures the concentration of acid. While there is a correlation between pH and acidity, they are in fact mutually exclusive. The concentration of acid can only be tested by way of a process called titration.
To illustrate this difference, two foods may share the exact same pH, yet the concentration of acid can be significantly different.
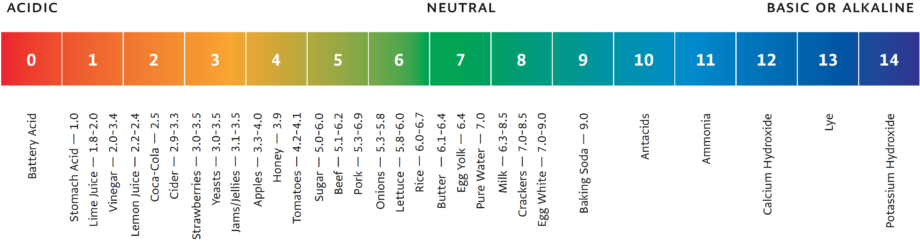
The Scale of Measurement
pH is generally measured on a scale of 0 to 14. Solutions with a pH of less than 7 are considered acidic; solutions with a pH greater than 7 are considered basic, or alkaline.
The Role of pH Measurement in Food & Drink
In relation to food and cooking, pH may be most commonly known as a measure to ensure food safety. For example, in water-bath canning, foods must have a pH below 4.6 to ensure safety and prevent growth of harmful bacteria.2 Foods with a low pH are resistant against dangerous microbiological growth and spoilage.
While food safety is a crucial consideration, understanding the pH of an ingredient can also help to achieve balance of flavors. Acidity, or sourness, is as essential as salt or seasoning in achieving balance of flavor, something all good chefs know.
Example Applications of pH Measurement in Food
The following are a few quick examples of the application of pH measurement in a variety of food products.
Quality of Meats
pH levels can be used to determine the quality of meats. For example, pork with a pH of 5.6 to 6.0 is indicative of a pig raised well and stress free, while pork with a pH of 4.9 to 5.5 indicates a pig raised poorly and/or stressed at slaughter.3
Salami
Meat used to make fermented products should be below pH 5.8.4 Salami should be fermented to pH 5.3 or below to protect against the growth of Staphylococcus aureus.5 pH can also be utilized to achieve and measure the desired sourness and resulting flavor in a finished product.
Cheese
pH offers an indication of contamination from bacteria or chemicals.6 Milk is typically in the range of pH 6.5 to 6.7. Values above this range may suggest illness in the cow, while values below this range may indicate that lactic acid fermentation has begun.7 Low pH will render a cheese devoid of shape, while high pH will produce a brittle, crumbly cheese.
Yogurt
Cultured milk must be brought to a pH value of 4.6 or lower before being cooled. Fruits added must be of the same pH, or the yogurt needs to be further acidified to compensate for more basic fruits.
Fermented Vegetables
In fermented vegetables, the growth of Lactic-acid bacteria can be gauged by pH measurements. Fermented vegetables need to reach a pH of 4.6 or lower to inhibit undesirable bacterial growth.
Wine
A pH between 3.0 and 4.0 is optimal for most wines. Understanding pH prevents spoilage by inhibiting microbial growth. pH levels are used to influence the appearance, aroma, and taste of wine.
Breads and Pastas
The acidity of the water used in baking affects the finished product. A slightly acidic (<7.0 pH) water is desirable. Batter should be acidified to pH 4.6 or lower, or kept under refrigeration, to ensure pathogens are not multiplied. Yeast prefer the slightly acidic conditions of a pH range of 4.5 to 6.0.
Litmus Paper
pH can be measured one of two ways: By litmus paper, (sometimes referred to as pH test strips) or by a pH meter and electrode.
Test Strips vs. pH Meter
Litmus is a water soluble mixture of different dyes extracted from lichens. Litmus paper test strips work by dipping the paper strip into a solution and comparing the change in color to a reference chart. The reference chart maps a color to a particular pH level: green-to-blue-to-purple for basic, and yellow-to-orange-to-red for acidic. Test strips are inexpensive, disposable and easily portable. They are a great solution for those on a tight budget where accuracy is not a factor of safety.
Manual Temperature Compensation
Measurement of pH is dependent on the temperature of the sample. As a temperature increases or decreases, ionic mobility increases and decreases respectively. The majority of test strips are standardized at 25°C (77°F). Compensation must be made for temperatures other than 25°C.
To manually calculate pH readings, use an adjustment of 1.9% pH per 1 degree Celsius above or below 25°C. For example, a test strip reading of pH 6.0 at a temperature of 24°C would be adjusted to an actual pH of 5.89; and a reading of pH 6.0 at a temperature of 26°C would be adjust to an actual pH of 6.11.
The Problem with Test Strips
In many cases, test strips do not provide the accuracy necessary. Measurement relies on visually matching colors to a printed reference chart, which assumes accuracy in the printing of reference colors. Further, the color difference in a 1 point range (e.g. pH 4 to 5) can be difficult to determine by eye. And to compound the issue, 1 in 12 men and 1 in 200 women have a certain level of colorblindness.10 In applications like water-bath canning, where a pH of 4.6 or lower is necessary to prevent c. botulinum toxin growth (botulism)2, a pH meter and electrode should be used, as test strips do not provide the precision required.
Tools for Measuring pH
Assuming that litmus test strips will not suffice, the following outlines mandatory and optional tools for a proper setup to measure pH.
Mandatory Tools
These tools should be considered necessary for a proper pH measurement setup.
- pH meter
- Electrode(s) (aka probe or sensor) (if not integrated or included with meter)
- Electrode fill solution (for re-fillable electrodes)
- Calibration buffer solutions
- Cleaning solution(s)
- Storage solution
- Deionized/Distilled water
- KimWipes®
Optional Tools
These tools should be considered optional. They are beneficial, but not strictly necessary.
- Glass beakers; 250-500mL and 50-100mL
- Laboratory wash bottle
- Magnetic stirrer
How a pH Meter Works
Foods which are acidic contain increasingly higher concentrations of positively-charged hydrogen ions. A pH-sensing electrode detects these positive charges and sends a positive voltage (mV) signal to the pH meter. The meter then compares the signal with a constant reference voltage from the reference electrode. 0mV indicates a neutral solution (pH 7.0) and positive mV values correspond to decreasing pH values (more acidic), while negative mV values indicate alkaline solutions—something that is more uncommon in foods.
Temperature Compensation
pH calculation is dependent upon the temperature of the sample. As such, temperature compensation is critical for accurate results. Bare-bones meters, like the Milwaukee MW101 lack automatic temperature compensation and require one to manually enter the temperature of the sample. Some, like the Milwaukee MW102, have automatic temperature compensation (ATC), but facilitated by way of a separate thermal probe. The best meters provide temperature compensation by way of a thermal probe integrated into the pH electrode.
Types of pH Meters
Meters can generally be divided into three categories:
Testers
Testers are typically entry-level pH meters which have integrated spherical glass-bulb electrodes. The most inexpensive testers, like the Hanna HI98107 tester, lack automatic temperature compensation and require manual calibration by way of calibration “screws”. Better testers, like the Hanna HI98128, have automatic temperature compensation and provide automatic one or two point calibration.
Portable Meters
Portable meters are designed to be hand held. Some are task-specific with a single-purpose electrode attached by a cable, while others allow for swapping out of separate electrodes for different applications. Meters like the Hanna HI99163, while designed specifically for meat (with a piercing tip), can be used for a wide variety of food testing applications.
Benchtop Meters
Benchtop meters are by definition less portable, yet often provide a much more robust set of features, like advanced data logging, conformation to Good Laboratory Practice (GLP), and interfacing with external computers. Most bench meters accept task-specific electrodes and some, like the Hanna HI2020, allow for measurement of other parameters such as DO (Dissolved Oxygen) or EC (Electrical Conductivity), using separate electrodes.
Advanced Features
Higher-end pH meters will have more advanced features, like electrode diagnostics that will inform you if the calibration buffers are contaminated, or if the electrode needs to maintenance.
pH Electrodes
Electrodes, also referred to as probes or sensors, come in a myriad of designs, most of which are application specific. To illustrate this, consider the kitchen knife: There are countless types of knives made for specific tasks. Take, for example, Japanese kitchen knives: The Gyuto, an all-purpose chef’s knife; the Honesuki, a boning and butchering knife; and the Yanagi is a single-bevel slicer, intended specifically for sashimi. You can cut a piece of fish with any sort of knife, but the outcome of one designed specifically for that task, like the yanagi, will result in a better experience,and often better outcome than that of a knife designed for a different task.That said, there is a good deal of flexibility within pH electrodes. For instance, the Hanna HI10480 pH electrode is designed specifically for wine. However, because it has a “clogging prevention system” it is equally well suited for other types of food or beverages (e.g. hot sauce or kombucha tea) which contain small particles that are prone to clog the junction of a typical electrode.
Filling Solutions
Electrodes are filled with an electrolyte reference solution, commonly referred to as the “fill solution”. Fill solutions can vary, but the two primary electrolyte solutions for food applications are potassium chloride and viscolene (a hard gel).
Types of pH Electrodes
Spherical-bulb, Glass Body Electrodes
Spherical-bulb, glass body electrodes are best suited for testing liquids. They are often less expensive than conical-tip, plastic (PVDF) body electrodes. These electrodes are typically filled with potassium chloride and contain silver chloride (AgCI). When an electrode is used, a small amount of the fill solution is exchanged through the junction into the sample. As such, the fill solution will be slowly lost over time. Most electrodes are refillable and many come with a bottle of fill solution, extending the life of the electrode. They also work for high-temperature testing (> 80°C / 176°F). Because fill solution is deposited in the sample, these types of electrodes are intended for samples that are discarded after testing.
Conical-tip, Plastic (PVDF) Body Electrodes
Conical-tip, plastic (PVDF) body electrodes are best suited for testing semi-solid samples. The conical tip makes it easier to penetrate semi-solid foods and provides better sample-to-electrode contact. Additionally, they are suitable for liquids, should a single all-purpose electrode be desired. They are used primarily for testing food and typically filled with a food-grade viscolene (hard gel) electrolyte. These type of electrodes are not refillable and have to be replaced over time, but also require no filling maintenance. Because the fill solution is “food grade”, they are designed for direct sampling of foods, like measuring the pH of a piece of raw meat that will be cooked and eaten.
pH Electrode Considerations
Double Junction vs. Single Junction Reference
A double junction electrode has an internal compartment surrounding the silver reference wire. These are typical in food-specific applications, as the inner junction protects the sample from silver contamination in potassium chloride-filled electrodes. Double junctions are also less prone to clogging, and thus have a longer life.
Open Junction vs. Ceramic Junction
The junction is the part of the electrode where electrolyte fluid is exchanged with the sample. The majority of food-specific electrodes will have what is referred to as an “open junction reference”. Food/beverage samples will often clog a conventional ceramic junction. Clogging can lead to erratic readings and frequent replacement. Conical tip electrodes are typically all open junction designs. Some spherical-bulb electrodes, like the Hanna HI10480 , have an open junction with an additional “sleeve” to protect against clogging from small particle found in turbid solutions like wine.
HACCP Compliance
There are no regulations that define a “HACCP compliant” pH meter or electrode. This type of classification is more a matter of marketing than one of actual regulation. That said, a conical-tip, plastic body electrode may provide the most flexibility when used in a HACCP program. While the levels of potassium chloride and silver chloride passed into the sample with a spherical-bulb electrode are inconsequential, electrodes filled with food-grade viscolene electrolytes are designed specifically for direct sampling of food, and are designated as “food safe”. Moreover, conical-tip electrodes provide greater flexibility as they can test solid (with piercing attachment), semi-solid and liquid foods.
Automatic Temperature Compensation
Higher-quality electrodes include integrated temperature sensors. Several manufacturers refer to this feature as “ATC”, or Automatic Temperature Compensation. Some lower-end meters include separate temperature sensors that are placed in the sample alongside the pH electrode.
High-temperature Measurement
Plastic, viscolene-filled electrodes cannot be used with samples above 80°C / 176°F, as the gel electrolyte will melt. Spherical-bulb glass electrodes can typically be used up to boiling 100°C / 212°F.
“Smart” Electrodes
Some electrodes include embedded microchips that store calibration data including date, time, buffers, offset, and slope of the last calibration. The smart electrode allows the user to change to another smart pH electrode for a different sample type without having to recalibrate. This is especially useful when using several different type of electrodes with a single meter.
Electrode Resolution
A resolution of 0.01 pH is sufficient for most food applications and typical of quality meters/electrodes.
Lifespan
The life of a pH electrode is not infinite. A number of factors can affect this: how often the electrode dries out and requires re-hydrating, how roughly it is used, lack of or improper cleaning, the higher the temperature of testing, or the more extreme the pH tested. A well-maintained electrode can last up to 2 years.
Calibration, Cleaning and Storage
The importance of proper calibration, cleaning and storage cannot be stressed enough. Pick any pH meter on Amazon and read the reviews. They are filled with stories of people relating their “problems” with a given pH meter. More often than not the issue is not one of a bad meter or malfunctioning electrode; rather, it’s one of misuse or user error. Probably the most common issue involves keeping the electrode properly hydrated in storage solution, as well as improper cleaning or infrequent calibration.
Calibration
Calibration represents the most crucial step in attaining an accurate pH measurement. Calibration ensures that the mV output of the pH electrode correctly correlates with pH values. All electrodes “age”. Calibration corrects for the changes resulting from electrode aging.
Calibration Buffers
Calibration is accomplished using calibration buffers, solutions that have an exact, known pH value. Buffers come in pH values of 1.0, 2.0, 3.0, 4.01, 5.0, 7.01, 9.0, 10.01, 11.0, 12.0, 13.0, and various other special values.
High-quality buffers come with a certificate of analysis. These are NIST traceable and contain a manufacturing and expiration date. Once a buffer is opened, the shelf life is accurate for around 6 months. Look for buffers with a five-year expiration as they are more resistant to degradation once opened.
Choosing a Calibration Buffer
It is recommended to calibrate with a buffer that brackets your expected sample. For example, if your sample is around pH 5.0, you would calibrate with 4.01 and 7.01 buffers. For most food applications, the 4.01 and 7.01 buffers will suffice. For more acidic foods, like vinegar or lemon juice, a lower buffer like 3.0 or even 2.0 may prove beneficial.
Cleaning
Just because an electrode looks clean, does not mean it is. The most common cause for pH inaccuracies is an unclean electrode. While the idea of paying for “special cleaning fluids” may seem unnecessary, it is in fact vital to ensuring the life of the electrode and accuracy of pH readings—and may save you money in the long term by extending the life of the electrode.
Types of Cleaning Solutions
Just as there are application-specific electrodes, there are application-specific cleaning solutions. A general cleaning solution will often suffice, but there are some scenarios where a special solution is necessary.
- General – Many food deposits can be removed and sanitized with a general cleaning solution
- Protein – For foods that are rich in protein (with the exception of meats) a protein-specific cleaning solution may be best
- Oil and Grease – Meats and foods like salami which have high fat content will leave oil and grease deposits. A cleaning solution specially formulated to cut these greases is necessary
The Importance of Proper Cleaning
When testing something like salami, fat deposits will accumulate on the electrode. Even when the electrode looks clean, there may be contaminants like a film of oil. An oil and grease cleaning solution uses an acid that removes the fat deposits and sanitizes the electrode 7qroo74.
A meter can still be calibrated even though the electrode is not properly cleaned before calibration. Calibration is then offset by the contamination. If the contamination dissipates after calibration, then the calibration is no longer valid and readings will be inaccurate.
Storage
The glass membrane and junction of the electrode must be kept hydrated at all times. Proper storage involves always keeping the protective cap or storage solution container filled with storage solution. Storage solution evaporates over time. If you only test occasionally, be sure to check periodically to ensure that the solution has not evaporated.
Hydration of the electrode is crucial to operation, as it maintains a hydrated gel layer on the testing surface , allowing for hydrogen ions to easily diffuse in and out. Storage solution also helps to keep the junction clean and clear, ensuring faster and reliable measurement.
Proper Storage Solution
Never store the electrode in distilled, deionized, or tap water, as this results in damage to the sensing glass and a shortened lifespan.
If the Electrode Dries Out
In the event that your electrode dries out, soak it in storage solution or 7.01 buffer solution for at least an hour prior to calibration or measurement.
Storage Solution Alternatives
In the absence of storage solution, a 4.01 or 7.01 buffer solution may be used for short-term storage.
How to Clean a pH Electrode
- Rinse the electrode with distilled water (a 250mL to 500mL beaker and laboratory wash bottle are ideal)
- Fill a 50mL or 100mL glass beaker with enough cleaning solution to completely cover the electrode tip and junction
- For general cleaning solutions: soak the electrode for 30m.
For protein cleaning solutions: soak the electrode for 15m.
For oil and grease cleaning solutions: rinse the electrode by swirling it in the solution - If contaminants remain, use a soft tissue (KimWipes® are ideal) soaked in the cleaning solution, and dab—never wipe—the electrode
- Rinse the electrode with distilled water
- Soak the cleaned electrode in storage solution for at least an hour before taking measurements
- Repeat cleaning procedure after measurement and always keep hydrated in storage solution
TIPS:
- Never wipe the pH electrode with a cloth or any other type of rough material. Only dab with a soft, lint-free tissue, like a KimWipe® soaked in cleaning solution, when necessary
- Never touch the electrode tip/bulb with your fingers or abrasive materials
- Salt deposits from the storage solution are normal and not cause for alarm
How to Calibrate a pH Meter Electrode
- If the electrode is dry, soak it in storage solution or 7.01 buffer solution for at least an hour prior to calibration
- Clean the electrode
- If using a spherical-bulb type of electrode with a liquid electrolyte, shake the electrode down, as you would a thermometer, to eliminate any air bubbles inside (if viscolene filled, this is not necessary)
- For refillable electrodes: Check the level of the fill solution. If lower than 1 cm (½”) from the top, top off with fill solution
- For refillable electrodes: Lower the rubber sleeve that covers the fill hole, or unscrew the fill-hole screw (this allows for faster readings)
- Prepare beakers: Use separate beakers for each buffer solution (50mL or 100mL are ideal) and a dedicated beaker for rinsing (a 250mL to 500mL beaker is ideal)
- Fill the two small beakers with the buffer solutions necessary to bracket your intended measurements; for most food applications, this will be 4.01 and 7.01 buffer solutions. Use enough solution to cover the tip and junction
- Prepare distilled or deionized water for rinsing (a laboratory wash bottle is ideal)
- Using the large beaker, rinse the electrode with the distilled water, then rinse with a small amount of 7.01 buffer solution to avoid diluting the buffer solution
- Enter the calibration menu of your meter, following the instruction to select the 7.01 buffer
- Submerse the pH electrode in the 7.01 buffer solution and wait for the meter to indicate that it is stable. Confirm calibration on meter
- Using the large beaker, rinse the electrode with the distilled water, then rinse with a small amount of 4.01 buffer solution to avoid diluting the buffer solution
- Submerse the pH electrode in the 4.01 buffer solution and wait for the meter to indicate that it is stable; confirm calibration on meter
- Using the large beaker, rinse the electrode with the distilled water
- Your electrode is now calibrated. Take your pH measurement or return the electrode back to the storage solution
TIPS:
- Always use fresh buffer solutions. Never reuse buffers
- Always clean the electrode before calibration
- If a high-degree of accuracy is required, calibrate your meter frequently
- If the meter takes an unusually long time to obtain a stable reading, the junction may be clogged and will require cleaning
- Salt deposits from storage solution are normal. Simply wash them off with distilled or deionized water
Direct Testing vs. Sample Testing
There are two primary methods for testing the pH in food:
Direct Testing
Some foods can be tested by taking a pH reading directly from the food product. For example, testing a salami, cheese, yogurt or wine. This is usually done with a conical-tip electrode with a plastic (PVDF) body. The electrode is inserted directly into the product and a measurement is taken. These electrodes almost always have food-grade electrolytes or double junctions, so the exchange of electrolyte or silver into the product is not a concern.
This type of testing only applies to samples that have uniform consistency and/or are not composed of liquid and solid parts.
Sample Testing
The second method of testing is by taking a portion or sample of the food, testing, and then discarding the sample.
Slurry Method
A form of sample testing, the Slurry Method, involves the dilution of a food sample with deionized/distilled water, referred to as creating a “slurry”. As pH is the testing of hydrogen ion activity, distilled or deionized water does not greatly affect pH readings, as there is not a significant amount of ions present.
Benefits
A slurry can be beneficial when you are testing a semi-solid or solid with a spherical-tip electrode, as it allows the sample to surround the electrode and read more accurately. Slurries can also be used when testing with litmus strips.
FDA Dilution Amount
The FDA suggests that a sample for pH testing not be diluted with more than 16.67% deionized/distilled water; that is, a 50g sample into 10g of water, representing a 5:1 sample-to-deionized water ratio.8
Practical Dilution Amount
Despite the FDA directive of no more than 5:1 dilution, samples can be diluted further without negative effect on the accuracy of the pH reading. We have tested dilutions up to 1:10 sample-to-deionized water ratio; that is, a 50g sample into 500g of water. The tests showed no significant difference when compared to lesser dilutions and/or direct testing.12 As for how far the dilution can be taken, this is wholly dependent on the composition of the sample being diluted. Some foods have low ionic strength and are difficult to test. Diluting these foods further only compounds the issue. As such, there is not a blanket “maximum” when it comes to dilution.
Recommended Slurry Ratio
That said, a 1:2 ratio of sample-to-deionized water (e.g. a 50g sample to 100g deionized water) should, in most cases, provide sufficient dilution to achieve a sample solution capable of testing with a spherical-bulb electrode.
How to Create a Slurry
- Prepare 50g of your semi-solid or solid sample
- Prepare 100g of deionized water
- Purée the sample with the deionized water until a uniform paste is achieved. If there is not sufficient liquid to create a thin paste, the sample may be diluted with up to 10x its weight with deionized water without affecting the pH level (e.g. 50g sample diluted with 500g water). Use as little water as necessary to achieve desired consistency.
- Test using the corresponding method that follows
pH Testing Methods
To ensure an accurate pH reading, follow the testing methods for the corresponding compositions of foods for which you are testing. These include: liquids, solids, liquid and solid mixtures, semisolids, and oil marinated foods.
The following methods are FDA-approved for testing food products of varied compositions,8 with the exception of the recommended dilutions for methods which contain a slurry, as previously detailed in Slurry Method.
Liquids
Example Foods:
Wine, beer, kombucha, vinegar, etc.
Testing Method
- Spherical-bulb or conical-tip electrode may be used. Ensure that the electrode is calibrated.
- Either pull a sample or test directly in the food product/batch
- Rinse the electrode with deionized or distilled water
- Submerge the electrode in the sample, stirring to ensure a consistent sampling (a magnetic stirrer is ideal); leave until a stable reading is reached, and record pH
- Rinse the electrode
- Take another reading and make certain that the second reading agrees with the first reading, as this ensures that the mixture is homogeneous
- Rinse and clean electrode. Return to storage solution
Solid Foods
Example Foods:
Raw meats, salami, cheese, etc.
Testing Method
- Conical-tip electrodes are best. Metal-blade piercing attachments may be beneficial. Ensure that the electrode is calibrated.
- Either pull a sample or test directly in the product
- Rinse the electrode with deionized or distilled water
- Insert the electrode in the sample; leave until a stable reading is reached, and record pH
- Rinse the electrode
- Take another reading and make certain that the second reading agrees with the first reading
- Rinse and clean electrode. Return to storage solution
Liquid and Solid Mixture Foods
Example Foods:
Canned products like pickles in brine, whole tomatoes in juice, etc.
Overview
Some products are composed of distinct solid and liquid parts. These parts often differ in acidity. Take for example, a jar of pickles. You have the solid part (the vegetable and spices) and the liquid part (the brine). To find the pH, you need to understand the equilibrium of these two parts. To do so, solid and liquid components must be puréed to a consistent paste before testing. (If liquid is an oil, see Oil Marinated Products)
Testing Large Batches of Liquid/Solid Mixtures
In a scenario where you have a large batch of pickles, you must strain and separate out all of the solids (vegetable and spices) from the liquid (brine). Record the weight of the solids. Record the weight of the liquid. If the solids weigh 10kg and the liquid weighs 5kg, the weight of the liquid is 50% that of the solids, a ratio of 2:1 solid-to-liquid. If you pull a sample of 3 pickles, with a weight of 300g, you would need to pull 150g (50%) in liquid. You would then purée the 300g of solids with 150g of liquid and test the resulting paste, which represents an equilibrium of the solid and liquid, in the same ratio as the larger batch.
Testing Method
- Spherical-bulb or conical-tip electrode may be used. Ensure that the electrode is calibrated.
- If the product is individually jarred, canned or bottled (e.g. a quart of jarred pickles) purée the entire contents of the product
- If pulling a sample from a larger batch, you must separate the solids from the liquids of the entire batch and determine their individual weights; once determined, you can pull a sample of the same solid-to-liquid ratio and purée for testing (see example above)
- Purée the liquid and solid components to a uniform consistency; if there is not sufficient liquid to create a thin paste, the sample may be diluted with up to 10x its weight with deionized water without affecting the pH level (e.g. 50g sample diluted with 500g water). Use as little water as necessary to achieve desired consistency.
- Rinse the electrode with deionized or distilled water
Submerge the electrode in the sample; leave until a stable reading is reached, and record pH
Rinse the electrode
Take another reading to make certain that the second reading agrees with the first reading. This ensures that the mixture is homogeneous
Rinse and clean electrode. Return to storage solution
Semisolid Foods
If consistency is uniform:
Example Foods:
Pudding, bread dough, etc.
- Spherical-bulb or conical-tip electrode may be used. Ensure that the electrode is calibrated.
- Either pull a sample or test directly in the product/batch
- Rinse the electrode with deionized or distilled water
- Submerge the electrode in the sample, leave until a stable reading is reached, and record pH
- Rinse the electrode
- Take another reading and make certain the second reading agrees with the first reading; this ensures that the mixture is homogeneous
- Rinse and clean electrode. Return to storage solution
If consistency is not uniform:
Example Foods:
Potato salad, egg salad, chunky tomatoes in sauce, etc.
- Spherical-bulb or conical-tip electrode may be used. Ensure that the electrode is calibrated.
- Pull a sample; do not test directly in batch
- Purée the sample to a uniform consistency; if there is not sufficient liquid to create a thin paste, the sample may be diluted with up to 10x its weight with deionized water without affecting the pH level (e.g. 50g sample diluted with 500g water). Use as little water as necessary to achieve desired consistency.
- Rinse the electrode with deionized or distilled water
- Submerge the electrode in the sample; leave until a stable reading is reached, and record pH
- Rinse the electrode
- Take another reading to make certain that the second reading agrees with the first reading. This ensures that the mixture is homogeneous
- Rinse and clean electrode. Return to storage solution
Oil-Marinated Foods
Example Foods:
Olives in olive oil, fish in oil, antipasto salad, etc.
Testing Method:
- Spherical-bulb or conical-tip electrode may be used. Ensure that the electrode is calibrated.
- Pull a sample
- Strain and separate the solids from the oil. Oil may be discarded, as it does not affect pH
- Purée the sample to a uniform consistency; if there is not sufficient liquid to create a thin paste, the sample may be diluted with up to 10x its weight with deionized water without affecting the pH level (e.g. 50g sample diluted with 500g water). Use as little water as necessary to achieve desired consistency.
- Rinse the electrode with deionized or distilled water
- Submerge the electrode in the sample; leave until a stable reading is reached, and record pH
- Rinse the electrode
- Take another reading to make certain that the second reading agrees with the first reading. This ensures that the mixture is homogeneous
- Rinse and clean electrode. Return to storage solution
Free pH Solutions
Hanna Instruments have graciously offered readers of Our Daily Brine free cleaning, calibration and storage solutions (a $50 value)
with the purchase of a qualifying pH meter.
References:
1. www.britannica.com
2. www.fsis.usda.gov
3. www.omafra.gov.on.ca
4. Handbook of Fermented Meats and Poultry, 536, GENERAL REMARKS ON THE PURCHASE AND SELECTION OF THE RAW MATERIAL
5. Handbook of Fermented Meats and Poultry, 331, PROCESS CONTROL POINTS FOR DRIED SA/USAGE MANUFACTURING
6. www.foodqualityandsafety.com
7. www.brewplus.com
8. www.accessdata.fda.gov
9. foodsafety.wisc.edu
10. www.colourblindawareness.org
11. www.fao.org
12. Hanna Instruments Slurry Testing; Nov. 15, 2015
Credits:
Special thanks to Justin Mecys and the entire team at Hanna Instruments, Inc., for the technical oversight, review, lab testing and general guidance throughout the process of writing this guide.
ALL RIGHTS RESERVED. No part of this guide may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or by any information storage and retrieval system, without written permission from the author, except for the inclusion of brief quotations when accompanied by a citation and link.
45 Comments
-
This is gold. I recently bought a ph meter and many questions and you wrote this guide. Thanks you so much for taking the time to write this out.
-
Author
Thank you. If you see any topics I missed, be sure to let me know.
-
-
This is a great article for salami makers. Isopropyl alcohol (rubbing alcohol) is a good cleaning agent for the electrodes when they are fouled by grease. Your comment about making slurries having no negative effect on the accuracy of pH measurement is incorrect in theory and in practice. A 1:1 dilution in theory will shift the pH by 0.3 units. In practice (I have done this myself 3 times), the non homogeneity of the sample gives unstable readings and fouled electrodes, sometimes showing this and sometimes showing a lesser pH offset. I would recommend a direct reading of the meat paste even if it requires multiple measurements and multiple cleanings.
-
Author
NCPaul, Thanks for your feedback. Regarding the slurry method, please check out these results. This is what we based the findings off of. There seems to be at most, 0.1 units in change, and on average less than that. You can see here that we have multiple dilutions (up to 10x) tested with multiple electrodes. Interested in hearing your thoughts after reading this. As for isopropyl alcohol as a fat cleaning agent, I have a request into the science team at Hanna to confirm. As far as I understand, it can be damaging to the electrode. I’ll confirm for sure and report back.
-
Hullo. This was very interesting but also a bit overwhelming. I want to be able to test pasta sauce and tomato soup to ensure I am not growing botulism. I need to have an oh of 4.6 or less.
I am just a domestic goddess so cannot justify a thousand dollars for fancy equipment. Do you have a recommendation as to which product will give me the most accurate results for a modest amount of outlay?
Kind regards
Julie Williams from New Zealand
-
-
Hello, great article. Full of useful information.
I wonder if you can give me a hand with the ph-meter selection.
Which of these ph-meters would you recomend for testing ph of sauces: Hanna HI98103 pH Tester, Milwaukee MW101 or Hanna HI98128?
I am a little confused on how the cleaning of these probes will be, since they have a plastic housing around the glass electrode. Would it store some sauce residues, would they be hard to clean?-
Author
Marian, Thanks for the kind words. As for the pH meter, I would probably point you at a totally different meter, if you have the budget. Out of those three, the Hanna HI98128 would be the best. The Milwaukee MW101 does not have ATC (reference article for this info), and would require you to use a thermometer to measure temp, then manually adjust the meter accordingly to the temp of the sauce you are testing. The pH Tester is a pretty entry level meter and better geared toward non-turbid fluids (read: not great for sauces). As you noted, they all have that plastic housing which is not ideal for cleaning. If you have an iPhone/iPad, you may consider the Hanna HI10482. It would be ideal for testing sauces as it has that little sleeve that prevents clogging of the electrode—and that’s something you’re going to encounter a lot in testing sauces with little food particles.The Hanna FC2022 would also be a great option, and may offer a bit more flexibility. It can be used to test more solid foods as well as sauces. As for cleaning, get that laboratory squirt bottle that I mention in the guide. It has a tiny spout and with pressure can give you a pretty nice stream to get those food particles clear. That combined with a good soak should do the trick.
-
Thanks for your answer.
-
-
-
Hey Kyle what’s up! Thank you for sharing your knowledge and all the tips! I am studying culinary arts and have found this very usefull for school work. I started curing and smoking at home and will continue experimenting, i am thankful for the help.
Saludos desde Panamá -
I require pH sensor which will sense pH oa a mixture which has vegetable crude oil,citric acid and casutic
-
Thank you for the great information my exhibition group will be very thankful for the greatinfo
-
Hi, I’m interested in knowing the proper ph levels for corn nixtamalization . Any idea on how to figure that out?
Love the article.-
Author
I’m sorry, that’s not something I have experience with. I’d suggest writing Hanna Instruments. They are very helpful and should at least be able to point you in the right direction.
-
-
Hi just started out so please excuse my ignorance, what recommendations for fermentation’s and drying out whole meat products and salumi
Regards
Glenn-
Author
Sorry, that’s not clear. What type of recommendations?
-
-
If I am looking to purchase a pH meter for home canning, what would be your recommendation? I’ve read of a few people using this one HI 98128W. Specifically, I can jams and jellies, pasta sauce and salsa, and also pickles. There are so many available that it’s a bit overwhelming!
-
Hey Kyle – first of all thanks for such a thorough guide, it’s super helpful in navigating the muddy waters of fermentation :) Question for ya! I recently made a fermented chili hot sauce, all looked good but I didn’t test the pH until today (7 days later). It’s at 4.3. I know the pH can change with time, but what is the likelihood it was higher last week before I tested it? Thanks so much!
-
I have noticed you don’t monetize your page, don’t waste your traffic, you can earn extra cash every
month. You can use the best adsense alternative for any
type of website (they approve all websites), for more info simply search in gooogle:
boorfe’s tips monetize your website -
Wow! This is amazing! Do you happen to have a list of food items you have tested and what their pH value is? I am a pediatric dentist trying to educate families on the importance of avoiding frequent ingestion of acidic foods and there is so much mis-information online about the true acidity of various foods that I am at a loss as to where to look for reliable values. Please let me know if you have some kind of index, tables, or spreadsheet that has actual pH values of commonly eaten foods. Thank you!!
-
Look at “Dropping Acid the reflux diet cookbook & cure” by Jamie Koufman and Jordan Stern, 2010. It has terrific data, tables of common foods ph, etc.
-
-
hello!,I love your writing so a lot! percentage we
communicate extra approximately your article on AOL?
I need a specialist on this space to unravel my problem.Maybe that is you! Having a look forward to look you.
-
Hi, Love this post! Can you link specific models that would work for testing sourdough starter and dough? Thanks!
-
Author
Yes. That’s the models with the food probe (green pointy) tip. You’ll see those mentioned here with links. Be sure to get a fat cleaning fluid. That sticky dough will clog the junction easily. You’ll need to clean that out every time.
-
-
Sorry Kyle, can you dumb it down for me? Which is the link I can use to buy one of the green tip ones please?
-
Author
The best way to go about this is to call Hanna. Tell them what you’re using it for and how and they’ll tell you best options. There are options. Not sure if you have a meter and just need an electrode, or a whole setup. How frequently it will be used. In what environment. Is HACCP logging required? Too many variables to point you to the perfect setup here. Call them. They’re very helpful.
-
Thank you!!!!
-
-
-
Hi Kyle, thanks for the great article! I entered my email for the “Free pH Solutions” but I still haven’t received any email or promo code. Any ideas?
-
Thanks for the awesome post. I am a bit confused about distilled vs. deionized water. I know the difference between them but I think the manuals or manufacturers usually mention deionized water but you also wrote distilled. If very accurate lab work is not the purpose, I am a home user interested in pH measurements for fermented food, is using distilled water ok for cleaning and preparing slurry ?
-
Hi, great and comprehensive article . I just want to vinegar pickle vegetables like my grandmother did. What meter do I need for a gallon jar?
Thank you! -
Wood furnishings possesses something incredibly
organic about it. There is this sense of warmth, of attributes and of beauty
that may be be located in hardwood furniture.Hardwood is actually birthed coming from the planet.
It feeds the fire, breaks down in to blows and ashes away.
It is actually very near to the human presence in the world.
Might be that is actually why it sounds so much with us.
May be that is actually why you still obtain that warm and comfortable emotion when you touch a rich
mahogany workdesk. -
What is the best brand benchtop (or portable) pH meter for high oil content products such as hazelnut paste? I am looking to buy one for our hazelnut processing company.
-
Author
Contact Hanna Instruments. I’d suggest the Edge for benchtop. They can point you to the best probe for oil. What’s most important, however, is that you get a good cleaning solution meant for cleaning oils from the probe. They will clog that little inlet easily.
-
-
so to measure pH level in fresh olives, how do you do it ?
also do you recommend measuring the brine level of the olives or this is totally different ?most important, is there a quick tool to measure it without worrying about buffers and other lab practices?
thanks
-
Author
To accurately measure I would take the entire contents of a jar, olives and brine, and puree it. Use the method described after that.
There’s no “quick tool” really. You need to calibrate, clean, store the probe well.
-
-
Question – when it comes to pairing things like Spirits and Premium cigars, or Coffee and Premium Cigars. Ph levels in the tobacco vary due to the soils in the different regions of the world it’s grown in. Spirits can also be considerably different. My question is there a method to prob inside a cigar (dry) and spirits (wet) to see what their Ph levels are?
-
Author
You could make a slurry of the cigar using DI water. Refer to that method. You may to let it set a while so the pH equalizes between dry and wet. I can’t say for certain, but that’s probably work well.
-
-
If we measure the powdery grain samples to measure the pH, how much mix ratio solid to liquid (distilled water) is ideal?
-
Author
I’m sorry but I don’t have experience with that. I’d assume enough to get an emulsion, but I just don’t know for certain.
-
-
How would you go about testing the PH of an assembled cake or cheesecake? Do you simply take the PH of the components (pastry/icing: crust/filling/topping), or would you use the slurry method?
-
I’m really interested in this topic! Mind asking what range of number would you consider as a good drinking water quality? I found that there are many controversy regarding this topic. Thanks!